Technology Driven

Vaccine Development
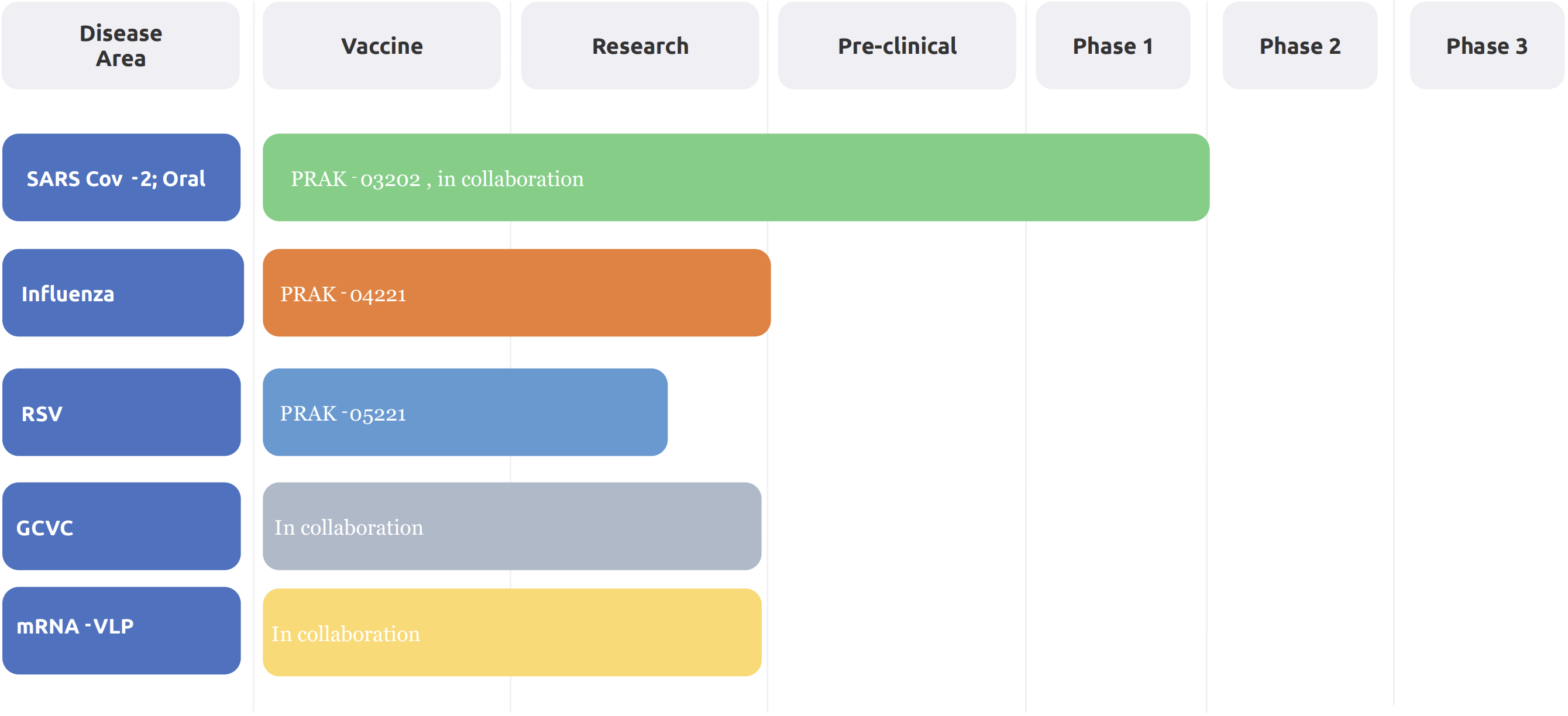
Post-pandemic, Premas has actively stepped into the development of vaccines that can combat future pandemics. On that path, Premas has developed a triple antigen vaccine candidate against SARS-Cov2, successfully completing Phase I clinical trials. It is the world's first triple antigen virus-like particle vaccine candidate, made using S. cerevisiae-based proprietary D-Crypt™ platform.
Vaccine Pipeline
Vaccine Development
Premas Biotech has initiated vaccine development, primarily utilizing its proprietary technology, the D-CryptTM platform, to deliver Virus-LikeParticles (VLPs) based vaccines for infectious diseases.
Premas is open to collaborations for the development of vaccines via multiple options of agreements.
Modality 1: Mutli-Antigenic VLPs (up to 3-4 antigens per VLP). Deliverables are the fully developed VLP.
Modality 2: mRNA-VLP: The mRNA-VLP is an exciting new area of vaccine delivery where the mRNA and VLP proteins may target the same infectious agent or dual modality. The mRNA-VLP complex is formed within the D-Crypt host, which acts as an assembly factory. Hence the production eliminates the complex CMC processes of RNA manufacturing
The mRNA=VLP is room temperature stable, via lyophilization and is rapidly scalable.
D-Crypt Platform:
Features
Advantages
Benefits
Premas Biotech has developed a multiple VLPs and tested the platform multi-modality development as well. The rapid development of VLPs against the infectious diseases include:
- Using the D-Crypt™ platform to deliver high producing hosts.
- Develop multi-antigenic VLPs, with up to 3-4 antigens.
- Develop highly cost effective and scalable processes
- Develop Analytical support data
- Develop Lyophilization
- Production of Drug Substance for clinical trials.
- Bulk Drug Substance delivery
- Dossier preparation
Process Development
Starting at 0.5L to 500L fermentation, we develop the process to suit the commercial manufacturing scales, along with a concomitant at scale, development of the clarification (broth) or lysis to release the product, and high-performance purification steps.
Products are either supplied in liquid form or lyophilized.
Clinical Manufacturing
We can also perform formulation and analytical development on the vaccine VLP candidates.
The DS can be released under a COA from Premas.
Analytics
Starting at 0.5L to 500L fermentation, we develop the process to suit the commercial manufacturing scales, along with a concomitant at scale, development of the clarification (broth) or lysis to release the product, and high-performance purification steps.
Products are either supplied in liquid form or lyophilized.
At Premas, various techniques are utilized to certify the identity, purity and contaminants loads on the final product. The test protocols and assays are:
- SDS electrophoresis, Immuno-blot
- Chromatography (SEC HPLC)
- Quantification by BCA or Bradford or A280.
- Develop potency & functional assays and physiochemical properties by immune assay (ELISA)
- Stoichiometry ratios of the individual proteins in the VLP
- Dynamic Light Scattering
- Moisture content by different orthogonal methods (by loss on drying and Karl Fischer method) (Lyo products)
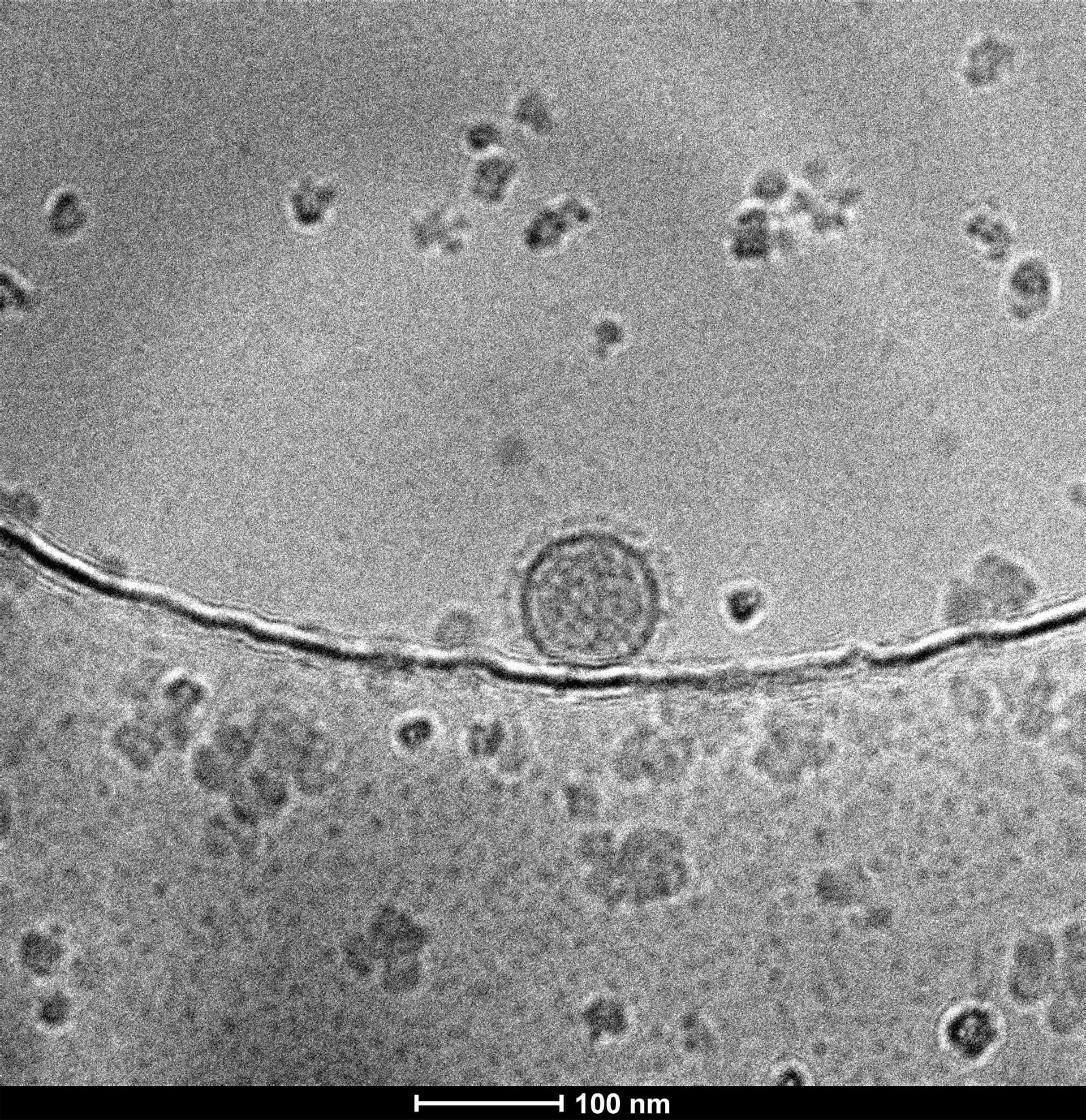
- TEM or Cryo-EM
- Mass Spectrometry
- Host Cell Proteins quantification
- Host Cell DNA quantification
- Endotoxins
- Bioburden (total plate count, amount of yeast and mold) ensures the product's safety.
Covid Vaccine
Developed Oral Covid Vaccine Candidate. The drug is in Phase 2 clinical trials initiation.
Premas has developed multiple vaccine candidates and their critical reagents for human and animal use. We are currently developing Influenza & RSV vaccine candidates for respiratory diseases.
Premas has also developed a recombinant carrier protein CRM-197 based on the E. coli expression system. Biophysical and functional characterization demonstrate comparability and bettering of effect with the existing commercially available molecules. CRM-197 is a proven carrier candidate for the success of glycoconjugate-based vaccines.
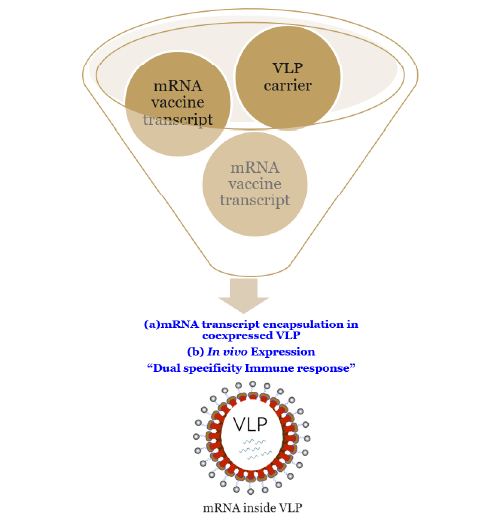
mRNA VLP
Different mechanistic approach for the delivery of the target molecule
Development of encapsulated circular mRNA
High expression yields
Increased shelf life
Induces direct immune response
Human like glycosylation
Encouraging POC data with high IgG response in mice immunogenicity studies
Platform amenability as optimized system to plug and play with
cargo-mRNA of choice
Induces direct immune response
Dual specificity immune response opportunities
Vaccine Advisory Board

Dr Tony D’Amore
Has a demonstrated history of working in the pharmaceutical industry. Skilled in Verification and Validation (V&V), Good Laboratory Practice (GLP), Vaccines, Validation, and GMP. Strong product management professional with a PhD focused in Biochemistry from the University of Windsor. Dr D’Amore retired as the Vice President, Product Research & Development, Sanofi Pasteur, after 28 years of distinguished service. He has been part of a number of vaccine initiatives.

Dr Gautam Sanyal
A highly respected vaccine researcher with a career that has spanned 38 + years, in various academic and pharmaceutical companies, namely, Mayo Clinic, Merck, Astra Zeneca, Medimmune, OSDD, and now has his own consulting company, Vaccine Analytics. Built and led biochemistry, protein science, bioanalytics, protein formulation, biophysics and structural biology departments within the pharmaceutical industry, aimed at characterising the structure, folding and function for both therapeutic and target proteins. He did his PhD from the University of Virginia, USA.

Professor Suman Das
Professor Das is the Associate Professor of Medicine at Vanderbilt University Medical Centre, Nashville, Tennessee, United States. He completed his PhD from ICGEB, New Delhi, India. He then moved to the United States and held multiple positions at NIH, Emory University School of Medicine, and over 10 years at the J.Craig Venter Institute, prior to joining his present position. Professor Das has significant experience in the field of virology and is considered a thought leader in his areas of research & expertise.
Publications
1.
PRAK 03202: A triple antigen virus like
particle vaccine candidate against SARS
CoV 2 (cell.com)
Saumyabrata Mazumder, Ruchir Rastogi,
Avinash Undale , et al. PRAK 03202: A
triple antigen virus like particle vaccine
candidate against SARS CoV 2.
Research article|volume7, issue 10,
E08124, October 2021
Published October 03,2021
DOI: https://doi.org/10.1016/j.heliyon.2021.e08124
Heliyon 7 (10) (October 2021) e08124
2.
Platforms, advances, and technical
challenges in virus like particles based
vaccines (nih.gov)
Gupta R, Arora K, Roy SS, Joseph A,
Rastogi R, Arora NM, Kundu PK.
Platforms, advances, and technical
challenges in virus like particles based
vaccines.
Front Immunol. 2023 Feb 9;14:1123805.
DOI : 10.3389/fimmu.2023.1123805.
PMID: 36845125; PMCID:PMC9947793
3.
Journal Vaccines
VLP - ELISA for the detection of IgG
antibodies against spike, envelope, and
membrane antigens of SARS CoV 2 in
Indian population (Just accepted)
