Point of care tests for SARS-CoV-2
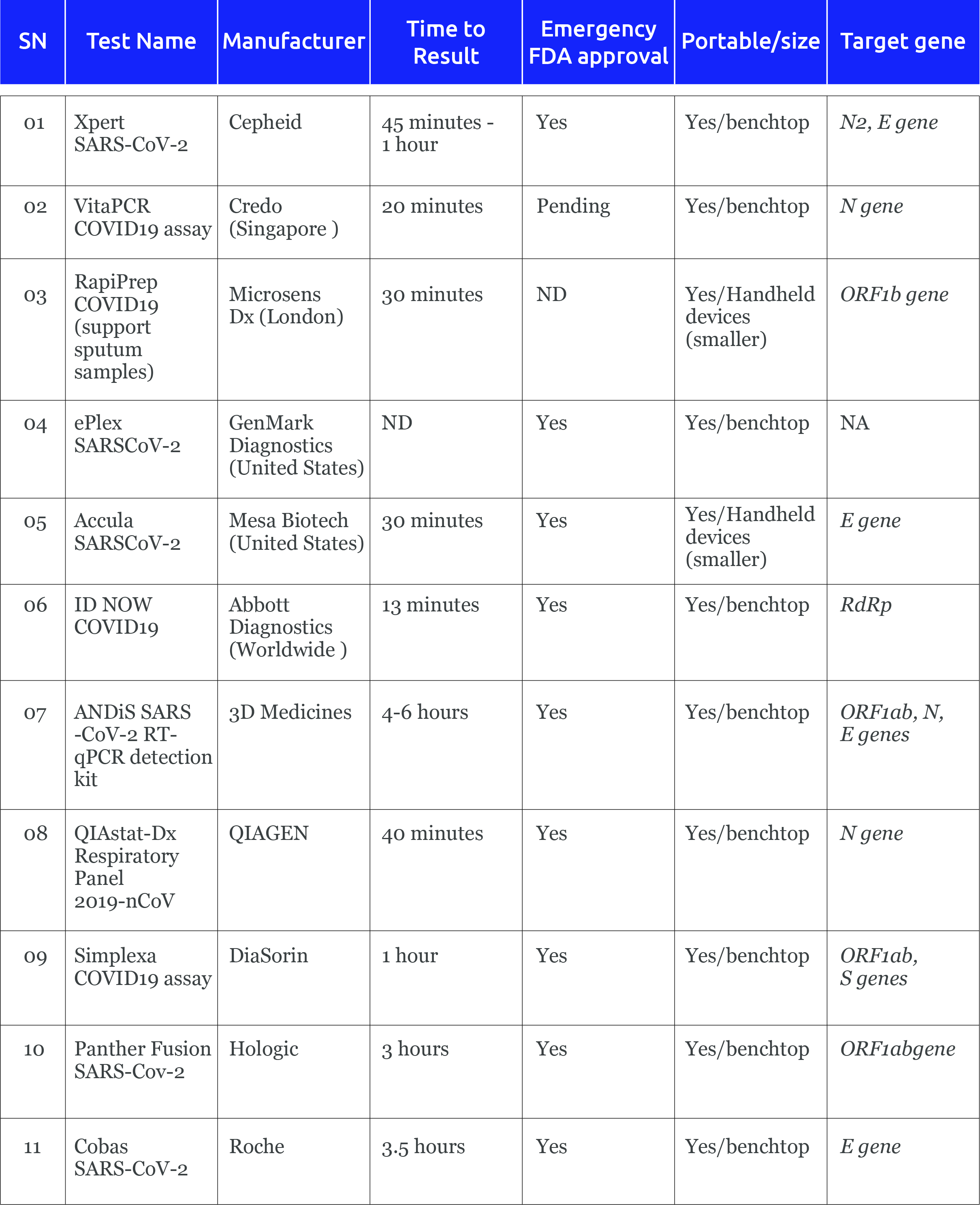
Molecular POC diagnostics
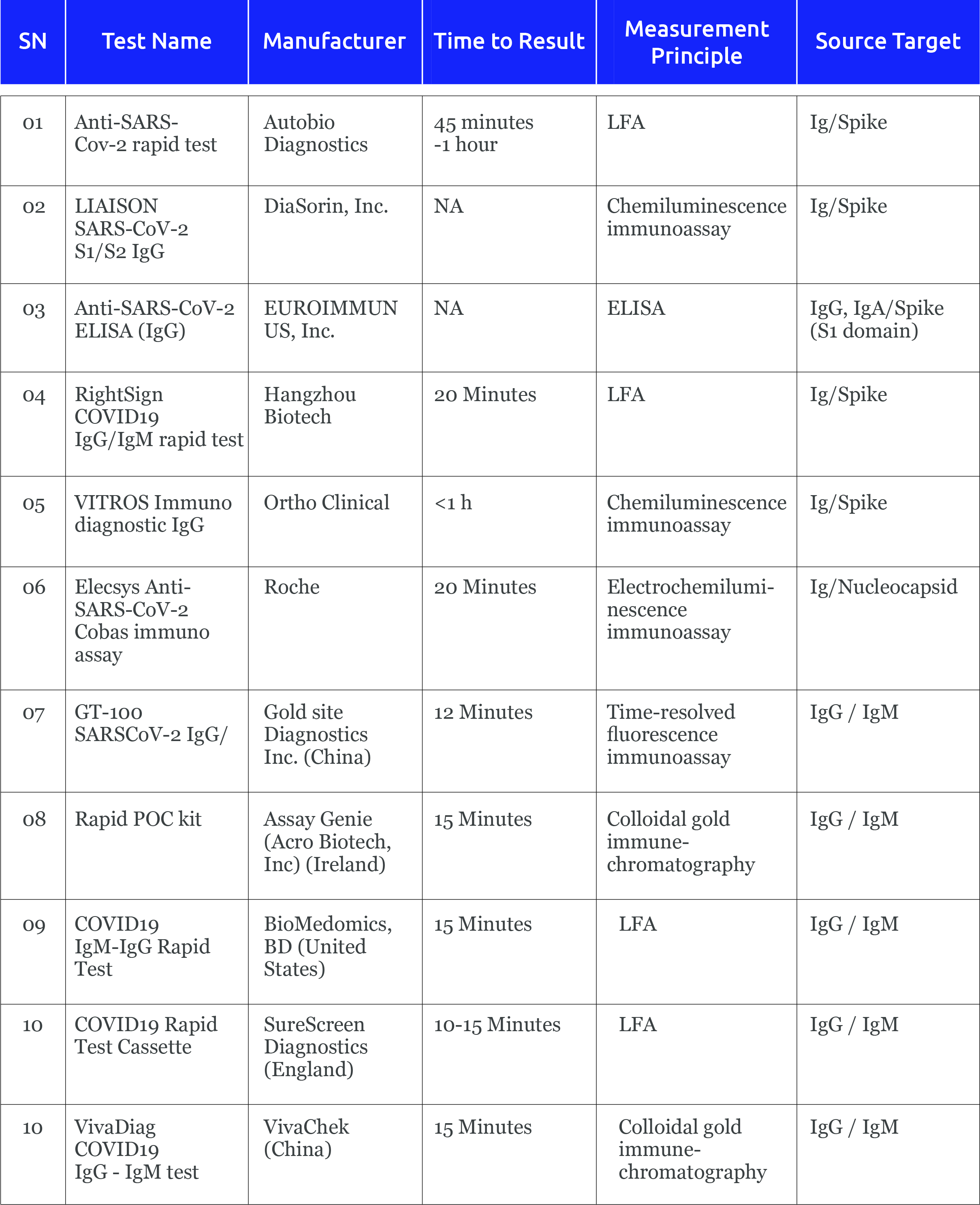
Antibody POC diagnostics
Limitations of serological tests
By Reeshu Gupta
Lead-Content Generation
10 December 2021