This would require you to make a decision based on your circumstances:
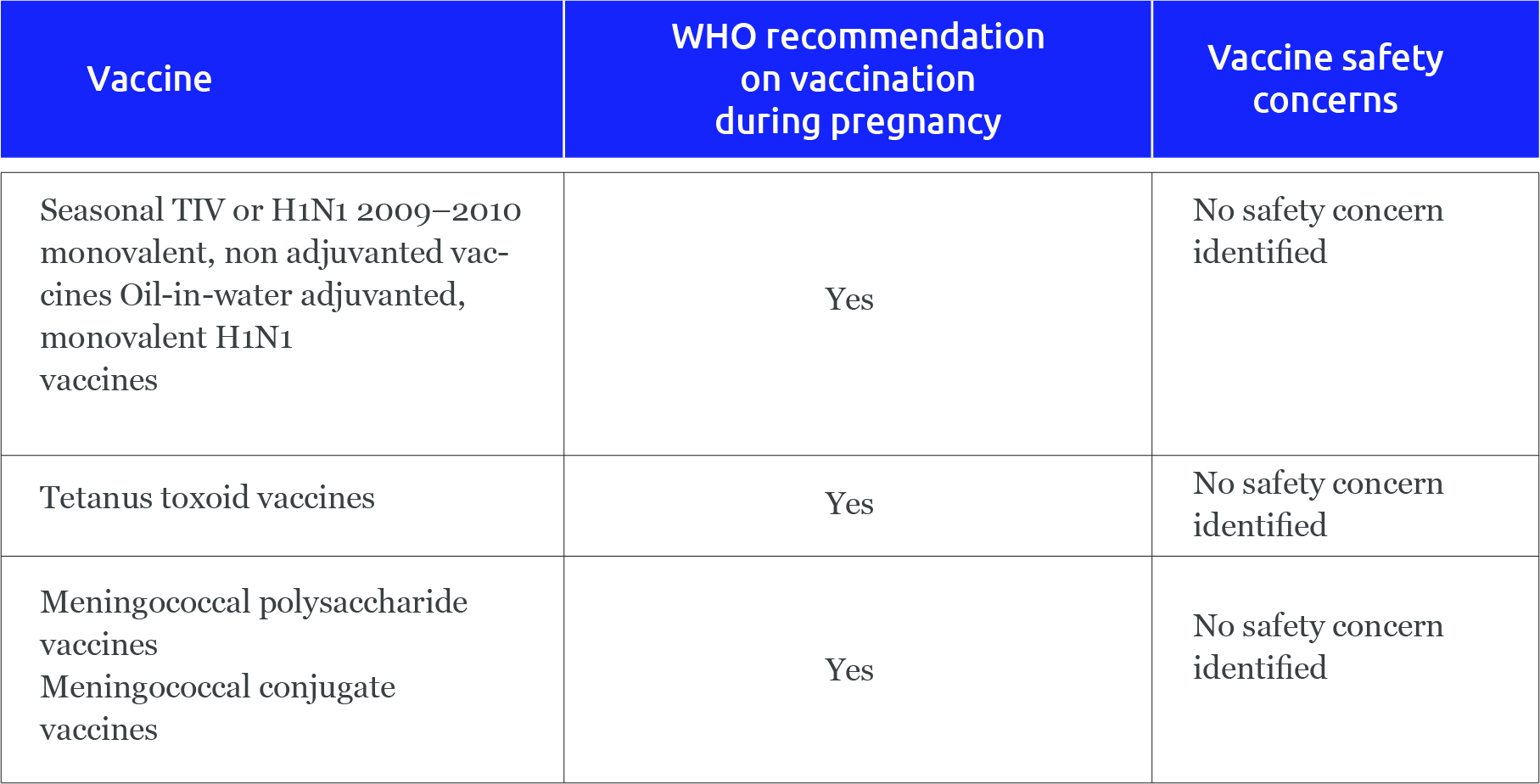
What we know about popular Covid vaccines:
Breastfeeding while you are COVID-19 positive:
By Reeshu Gupta
Lead-Content Generation
03 June 2021



