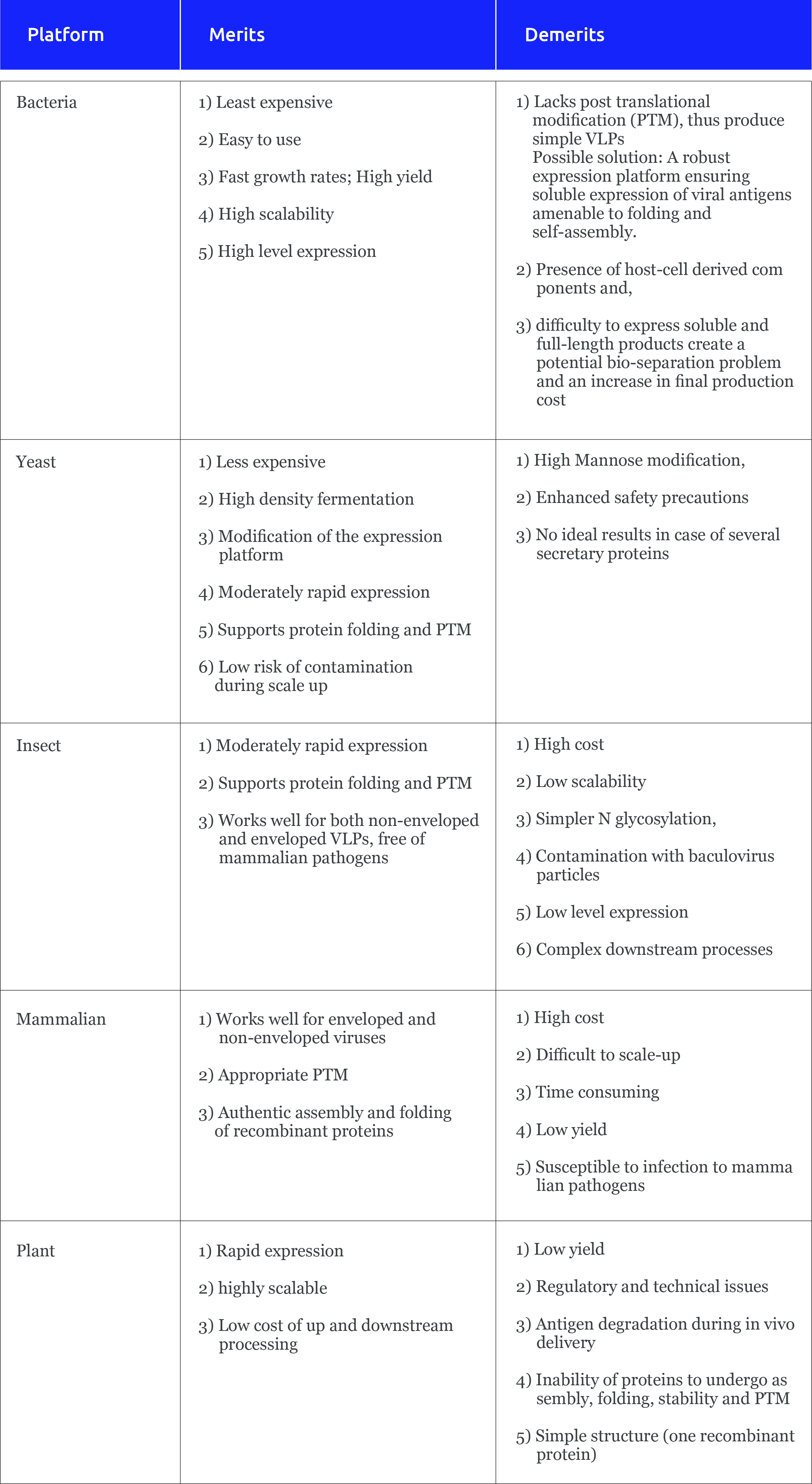
Expression platform for VLPs
D-crypt™
By Reeshu Gupta
Lead-Content Generation
24 October 2021
Recommended reads

7 Reasons why Covid-19 Vaccine Acceptance is Disappointingly Low?
In 2020, the World Health Organisation (WHO) listed reluctance to vaccinate despite their availability as one of the top ten global health threats. As per the report, about 80% of people worldwide think vaccines are both safe and effective. In contrast, the remaining 20% of people are worried and can potentially influence others.
Read More