Background:
Problem/Rational:
Solution/Approach:
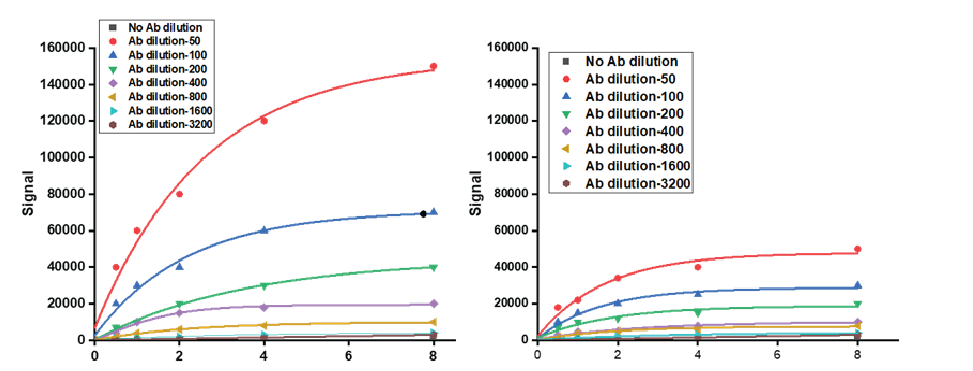
Fig 1: ELISA demonstrating activity of rotavirus glycoprotein
Measurable impact:
Recommended reads
Expression of Highly Active Protein in E. coli for Diagnostics Applicatio
The production of recombinant enzymes has led to the development of many diagnostics strategies in various medical fields. The increasing demand for recombinant proteins has created the need to develop efficient protein expression techniques to increase the productivity of the product.
Read More
Expression of Nav1.7
Voltage-gated sodium channels are transmembrane proteins responsible for propagating the electrical signal in neurons, cardiac, skeletal muscle, and neuroendocrine cells. Sodium channel Nav1.7 is preferentially expressed in peripheral neurons. Nav1.7 is a validated target for pain treatment in humans. The principal subunit of this channel is a protein of >200 kDa, the alpha subunit, which consists of four large domains of internal homology. The subunit forms the pore and functions in voltage-dependent activation and inactivation,
Read More
Expression of Full-Length Neuraminidase (NA)
Below is the case study describing the expression of full-length neuraminidase in the protease-deficient strain of Baker’s yeast, S. cerevisiae of D-Crypt™. The platform provides an ideal eukaryotic environment for expressing recombinant proteins retaining their correct structure, function, and post-translational modifications resembling those of humans. We used an array of proprietary expression vectors with designed upstream sequences for enhanced expression of neuraminidase.
Read More