Background:
Problem/Rational:
Solution/Approach:
Strategy for Purification:
Purification Procedure:
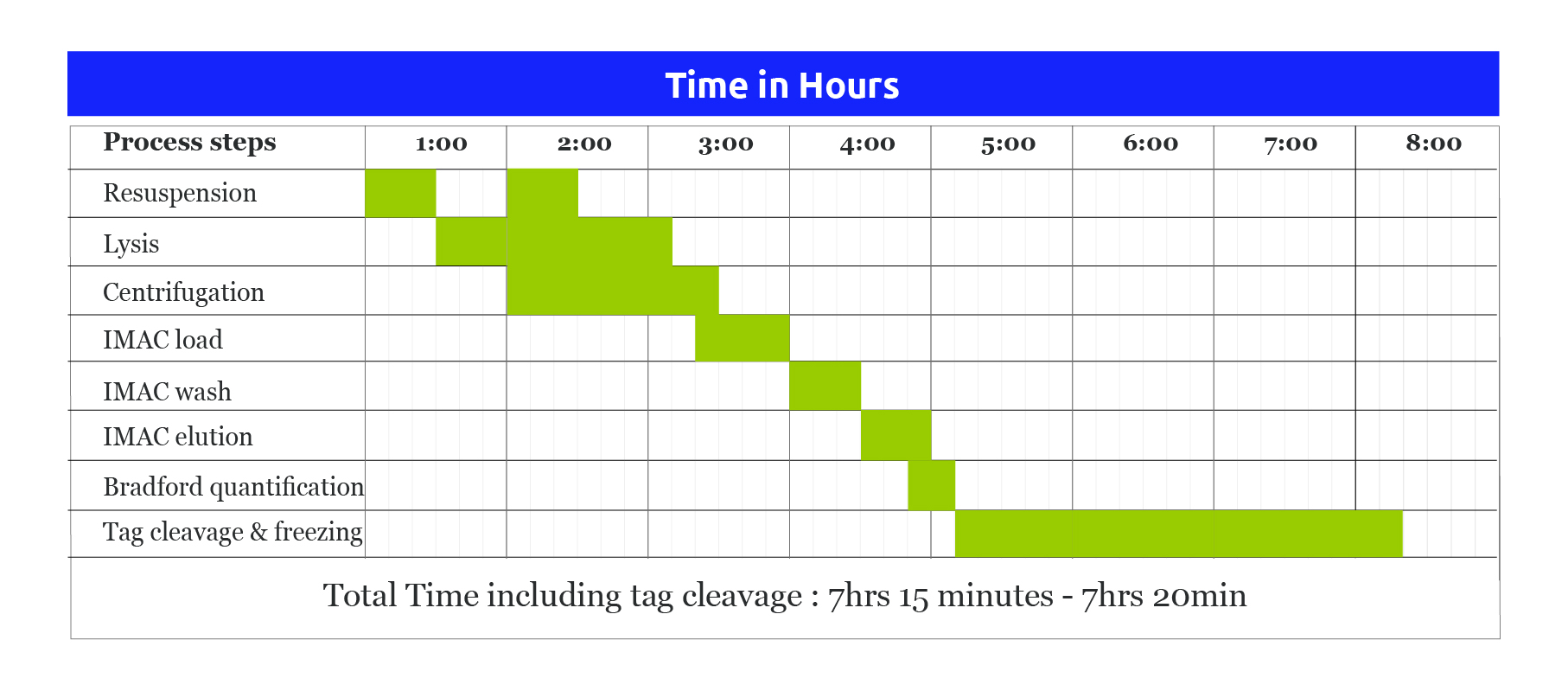
Fig.2 Procedure for Purification of protein
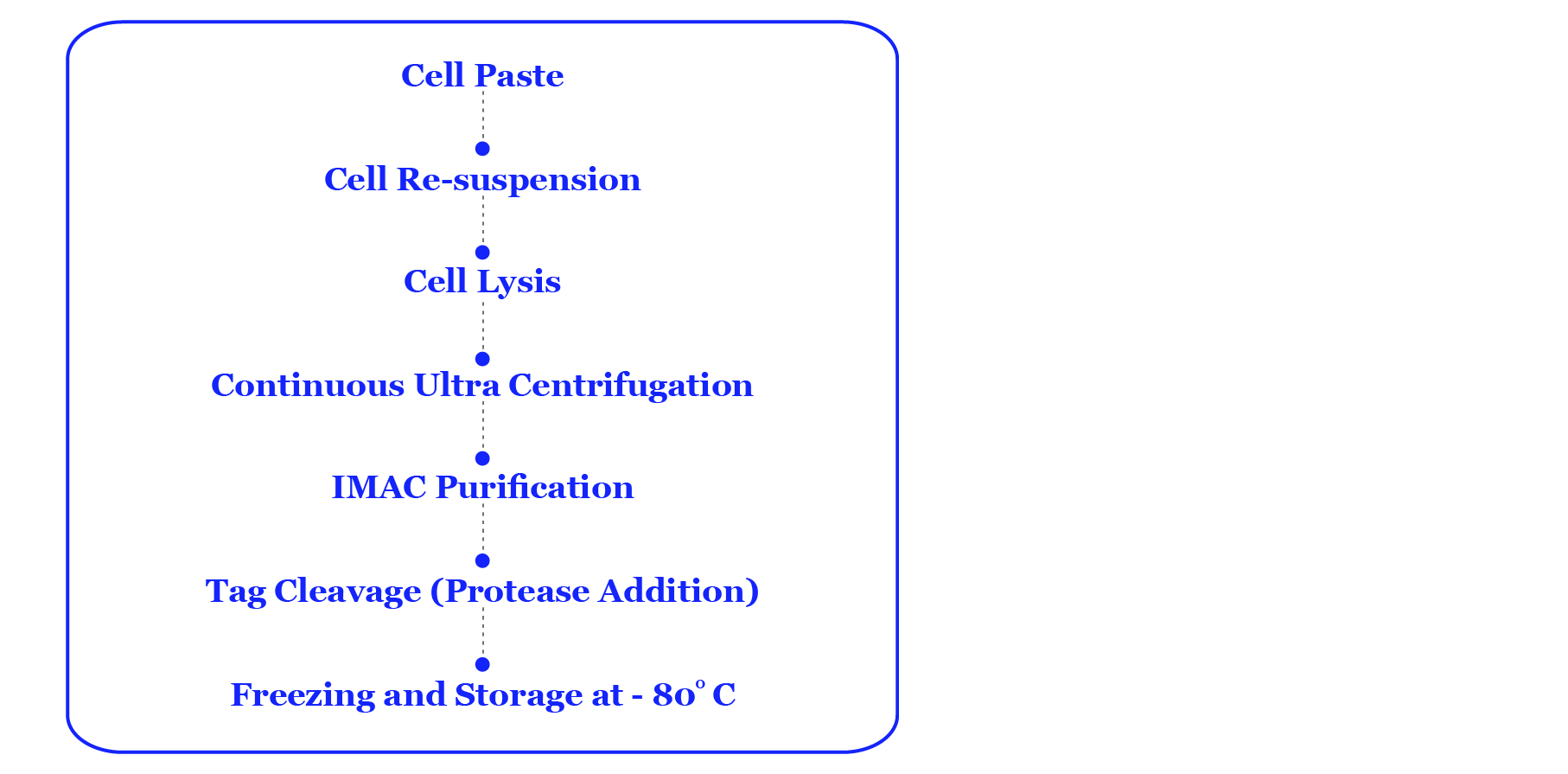
Fig 3: IMAC purification profile
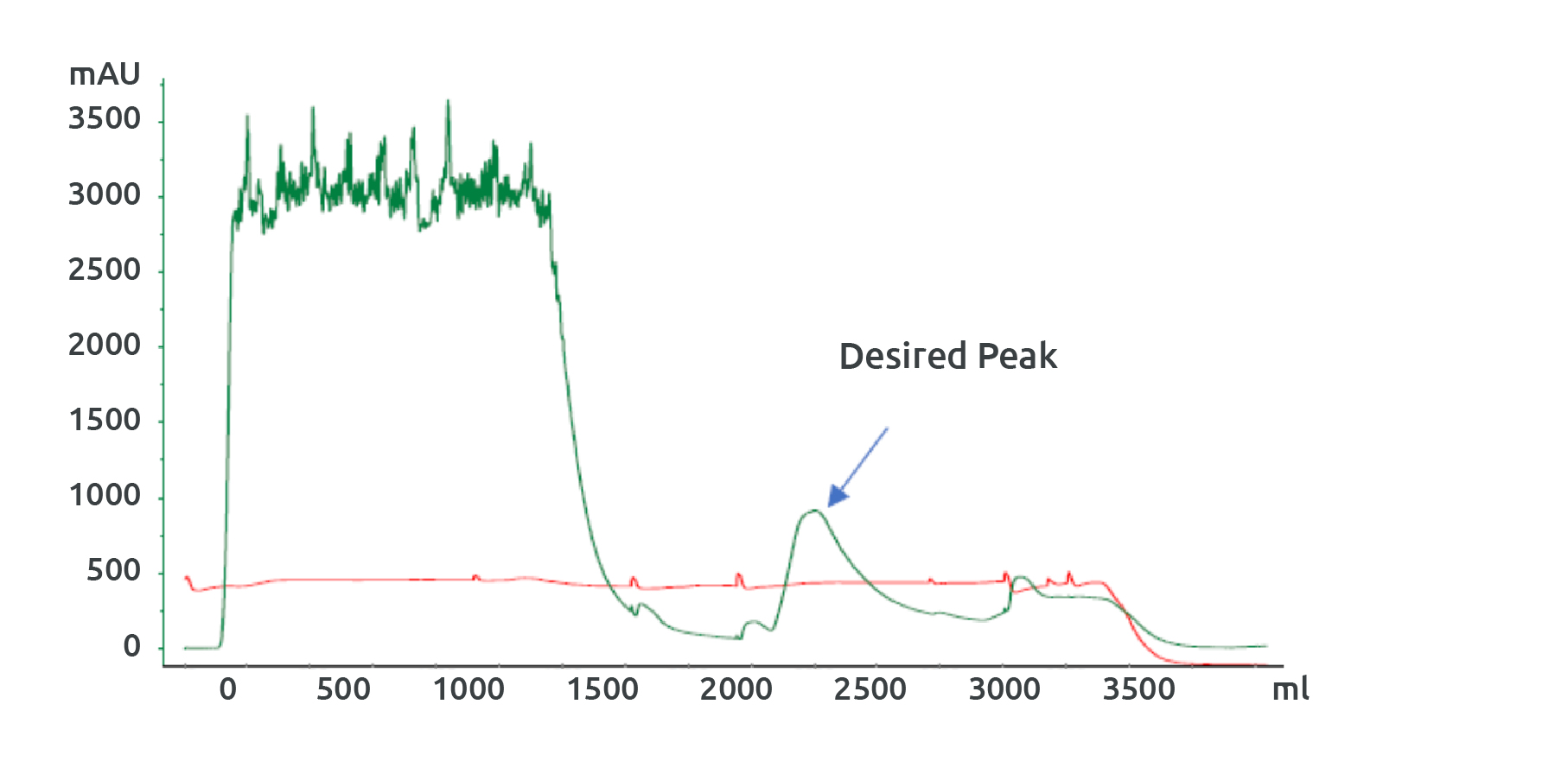
Fig 4: Protease cleavage of NusA tag A) Before protease addition B) After protease addition
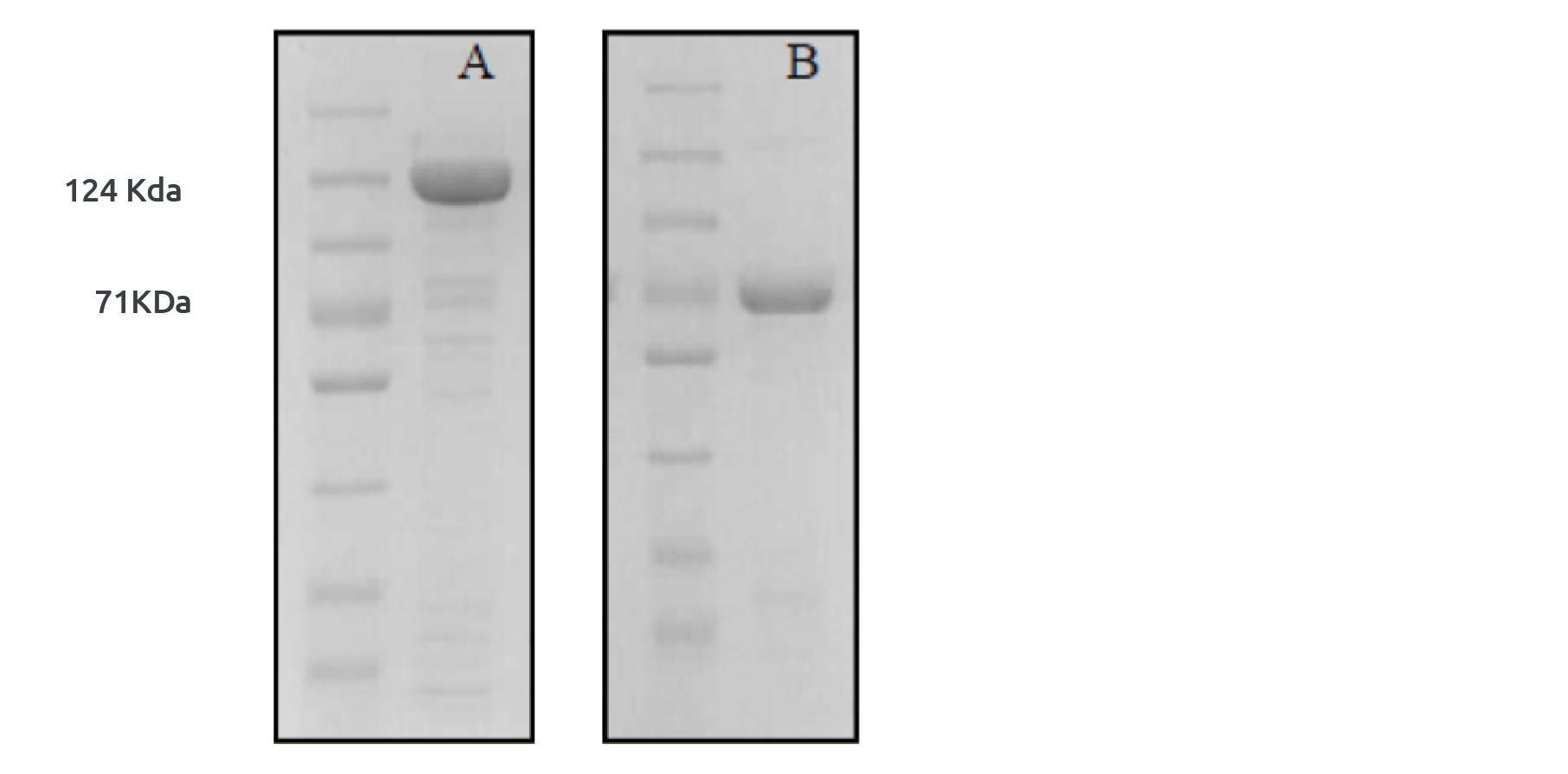
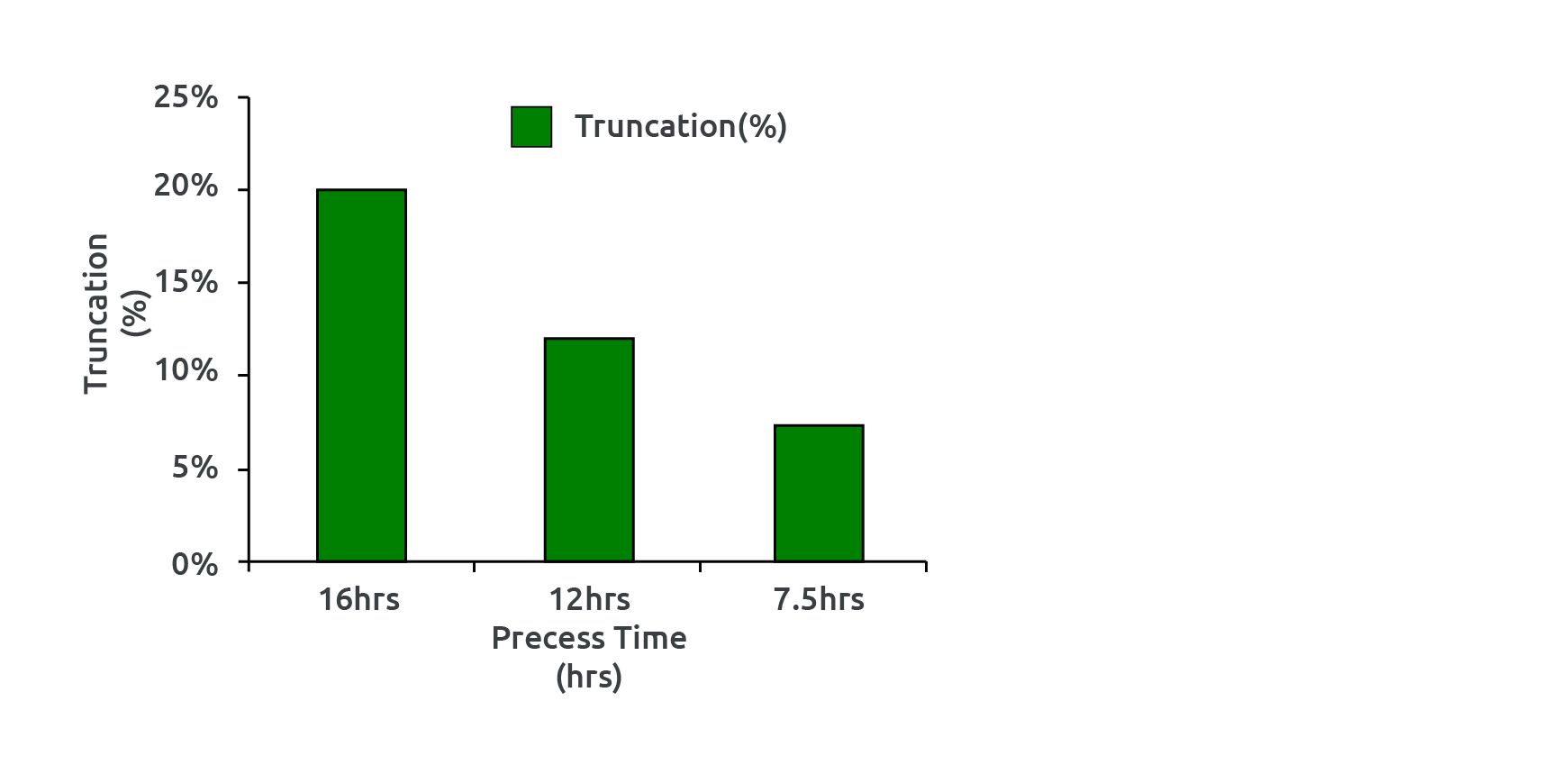
Fig 5: Change in percent truncation with reduced processed time
Measurable Impact:
Recommended reads
Expression of Nav1.7
Voltage-gated sodium channels are transmembrane proteins responsible for propagating the electrical signal in neurons, cardiac, skeletal muscle, and neuroendocrine cells. Sodium channel Nav1.7 is preferentially expressed in peripheral neurons. Nav1.7 is a validated target for pain treatment in humans. The principal subunit of this channel is a protein of >200 kDa, the alpha subunit, which consists of four large domains of internal homology. The subunit forms the pore and functions in voltage-dependent activation and inactivation,
Read More
Expression of Highly Active Protein in E. coli for Diagnostics Applicatio
The production of recombinant enzymes has led to the development of many diagnostics strategies in various medical fields. The increasing demand for recombinant proteins has created the need to develop efficient protein expression techniques to increase the productivity of the product.
Read More