Background:
Rationale:
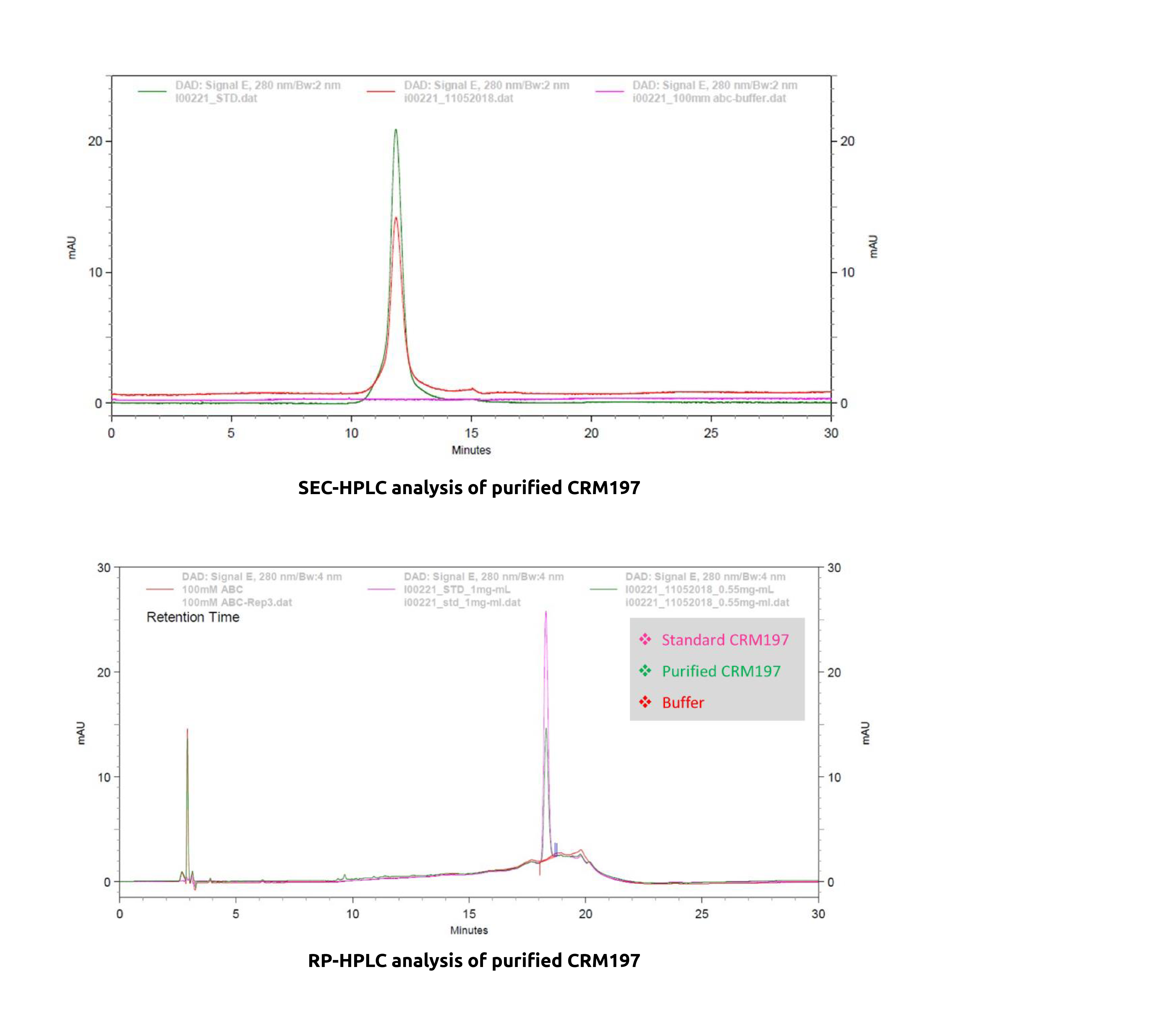
Approach:
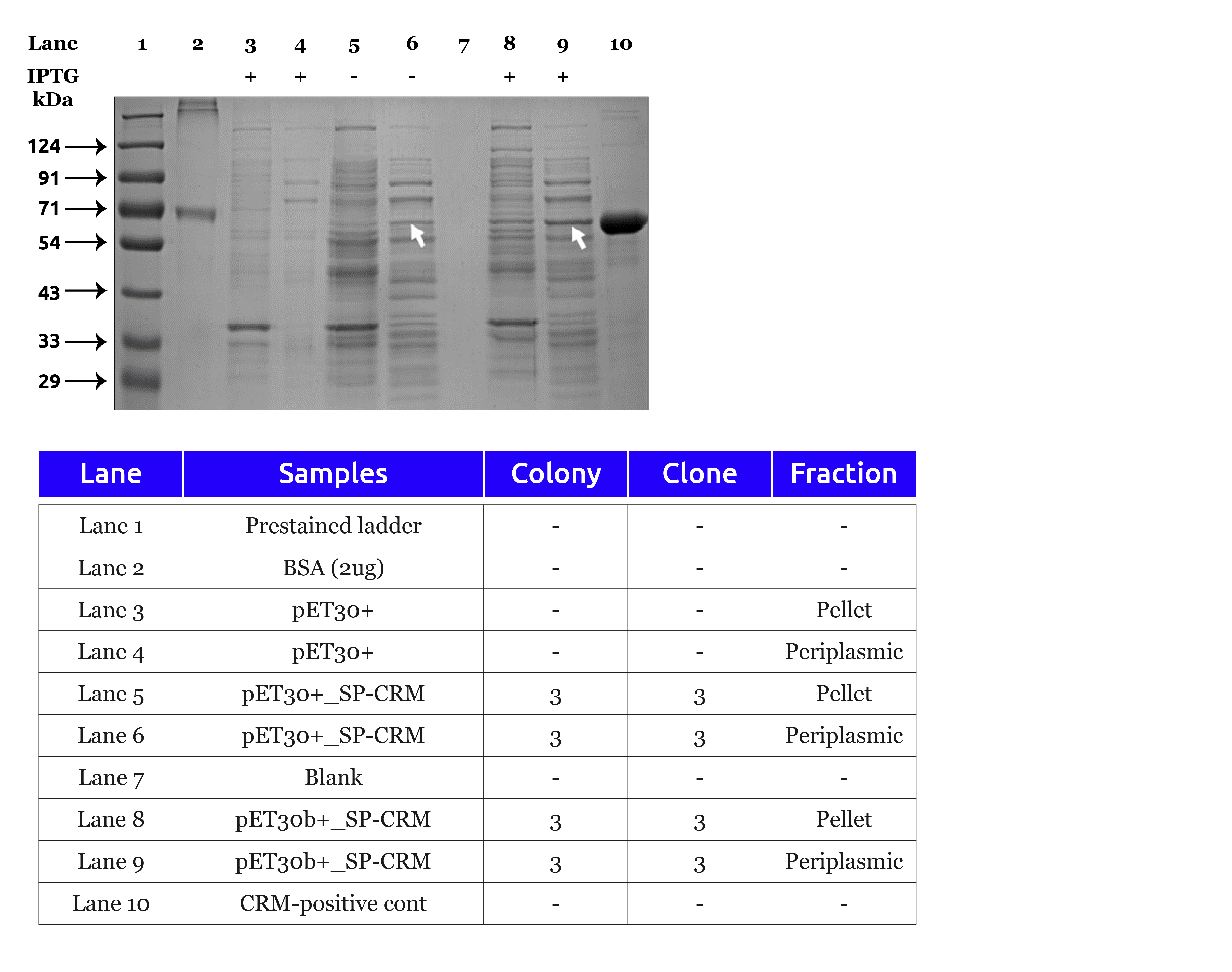
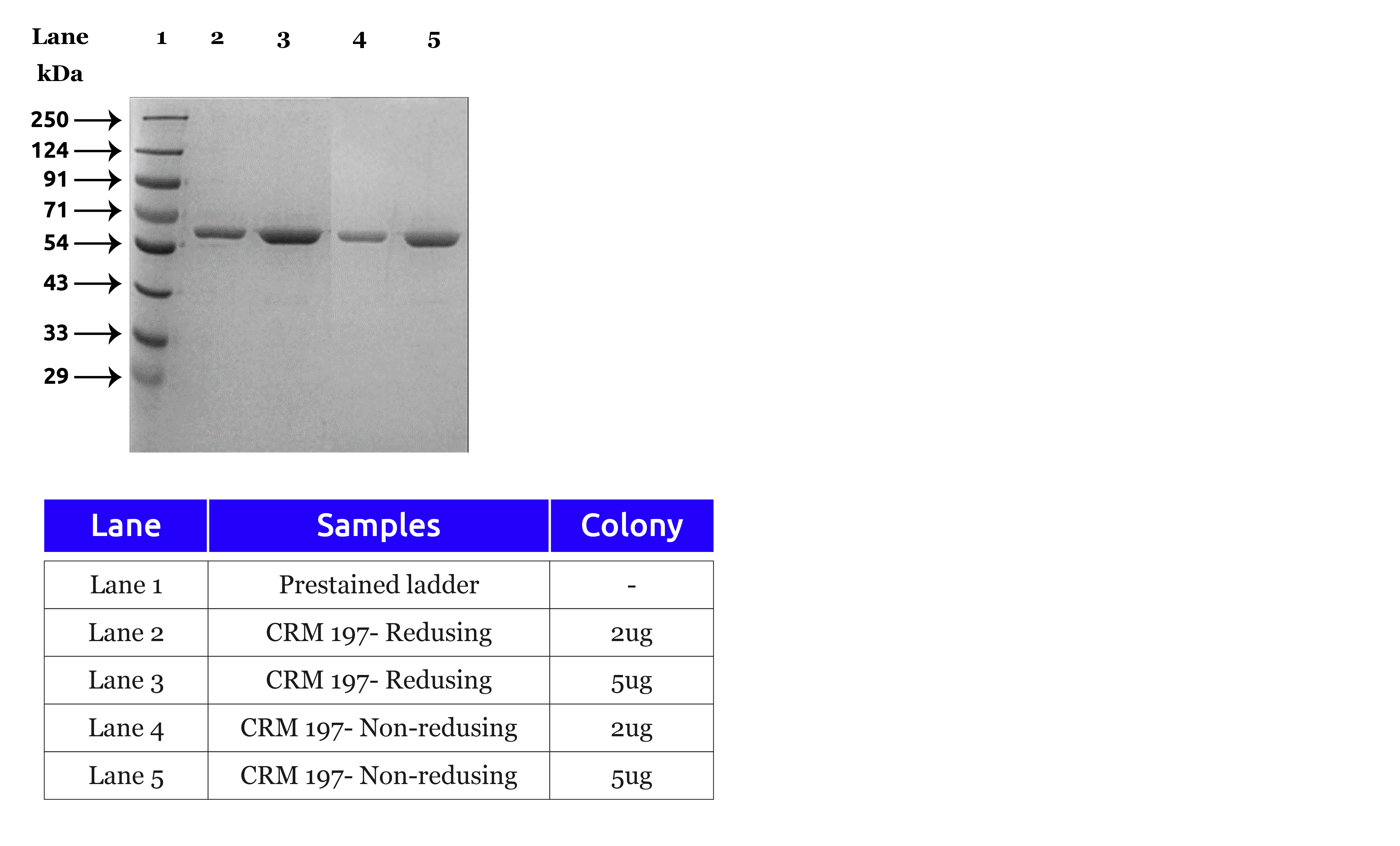
Measurable Impact:
Recommended reads
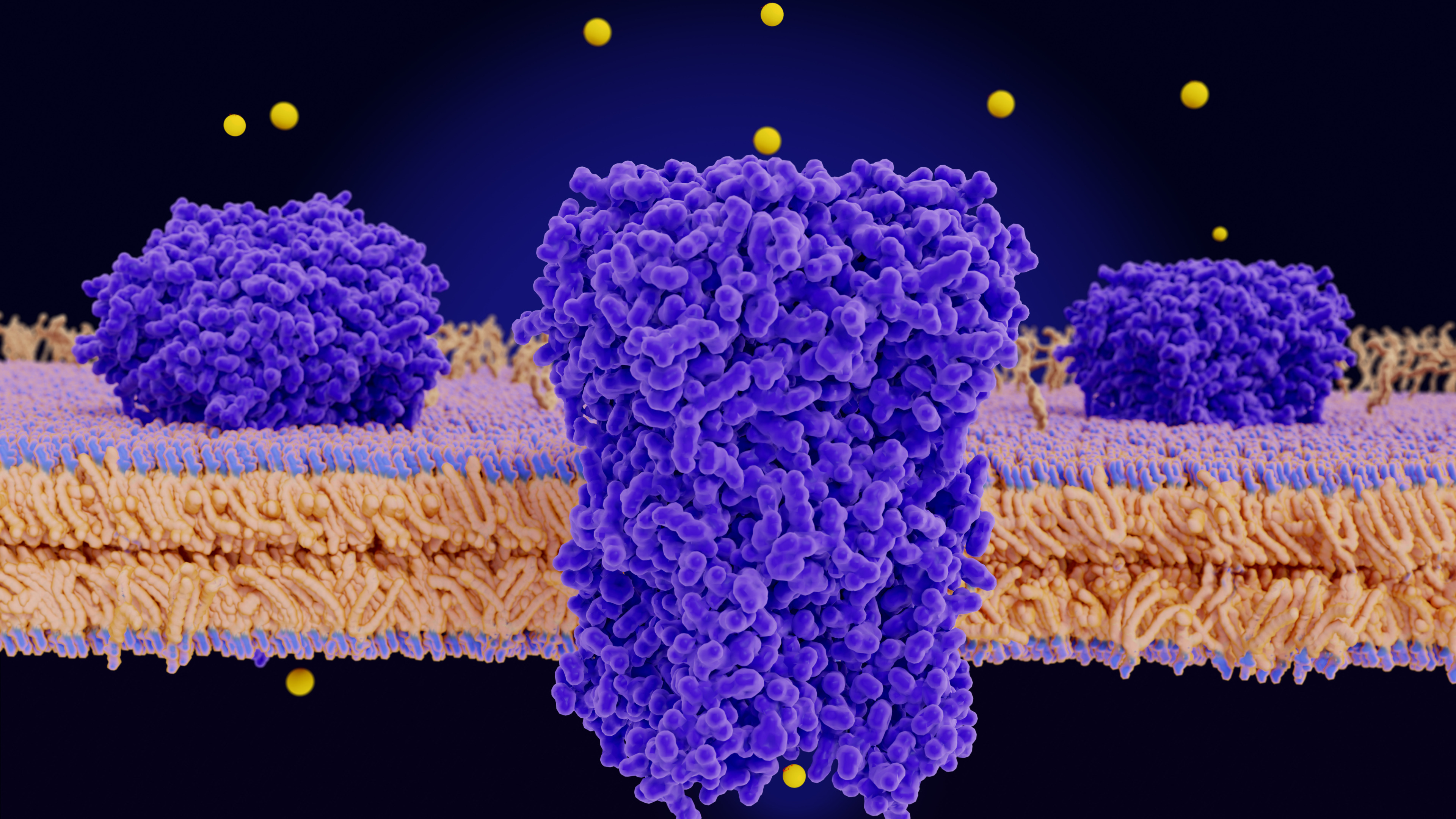
Expression of Nav1.7
Voltage-gated sodium channels are transmembrane proteins responsible for propagating the electrical signal in neurons, cardiac, skeletal muscle, and neuroendocrine cells. Sodium channel Nav1.7 is preferentially expressed in peripheral neurons. Nav1.7 is a validated target for pain treatment in humans. The principal subunit of this channel is a protein of >200 kDa, the alpha subunit, which consists of four large domains of internal homology. The subunit forms the pore and functions in voltage-dependent activation and inactivation,
Read More
Purification Optimization and Large-Scale Production of a Recombinant Protein fr
The recombinant protein is a shortened form of the native isolate, requiring multiple tests and regulatory submission characterization. Some bacteria produce toxins that show specific activity against certain insect species. This specificity is mainly due to a unique binding site in the insects' midgut epithelium membrane, where the protein inserts and causes damage to the wall.
Read More